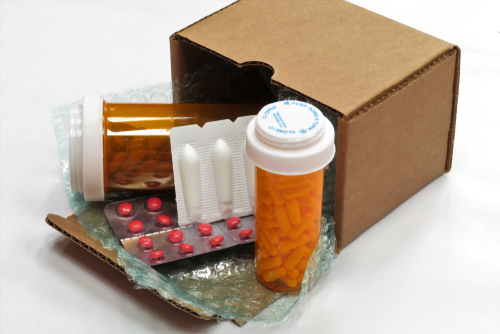
The Supreme Court is expected soon to decide — indeed, may have decided by the time you read this — whether to let stand or review a lower court decision clamping down on mail-order abortion. The focal point of the dispute is the widely used abortion drug mifepristone.
Mifepristone is used in more than half of all U.S. abortions. The drug causes abortion by breaking down the lining of a pregnant woman’s uterus.
Seeking review of a 5th U.S. Court decision imposing restrictions on the drug are the Food and Drug Administration (FDA), the U.S. Department of Justice, and Danco Laboratories, its manufacturer.
A group called the Alliance for Hippocratic Medicine, made up of pro-life physicians and others, claims the FDA, acting under pressure from the administrations of former president Barack Obama and President Joe Biden, cut corners to make mifepristone more easily available. (The group’s name is a reference to the oath, named for the Greek physician Hippocrates, by which physicians pledge themselves to practice medicine ethically.)
The case (Food and Drug Administration v. Alliance for Hippocratic Medicine) comes to the Supreme Court on appeal from a ruling last August by a three-judge panel of the 5th Circuit Court upholding a district court decision that placed substantial restrictions on the drug. The Supreme Court had earlier refused to consider the case until the 5th Circuit Court ruled on it.
If the Supreme Court declines to take up the case, it will be a victory for the pro-life side in the dispute. If it agrees, that will leave the outcome suspended until it hears and decides the case. A minimum of four justices must vote to hear a case for it to be accepted for review.
The FDA first gave its approval to mifepristone in 2000, then in 2016 and 2021 relaxed restrictions on it, allowing its use up to the 10th week of pregnancy — the point at which an unborn infant has a beating heart. The FDA also permitted nonphysician health care providers to prescribe the drug, and allowed it to be prescribed and sent through the mail without an in-person examination.
The ruling by the 5th Circuit Court of Appeals — which covers Texas, Louisiana, and Mississippi — reduces the time frame from 10 weeks to seven, the point at which the fetus begins to feel pain. It bars nonphysician prescription of mifepristone and sending it through the mail, and requires three in-person visits by the woman to the prescribing doctor.
A fact sheet on chemical abortion prepared by the Secretariat of Pro-Life Activities of the U.S. Catholic Bishops’ Conference notes that the FDA, when approving chemical abortion in 2000, also issued “strict protocols” for the protection of the mother. The original safeguards were later incorporated in a Risk Evaluation and Mitigation Strategy adopted in 2011.
But, the fact sheet says, the safeguards were “severely weakened” in 2016 when the FDA began permitting nonphysicians to dispense the drug and reduced the number of office visits required from three to one. Then in 2021, it says, the FDA began allowing mail-order abortion and eliminating “any pretense of” a doctor-patient relationship. “With this action,” the fact sheet adds, there is no knowing “the full extent of harm to the women whom the FDA is supposed to protect.” Among the potential problems it lists are serious injury and even death resulting from complications like previously undetected ectopic pregnancy, severe blood loss, and other problems.
And all to what end? “In addition to being a profitable new ‘product,’ ” the USCCB fact sheet says, “telehealth, mail order, at-home, do-it-yourself chemical abortions are a boon to the abortion industry.”