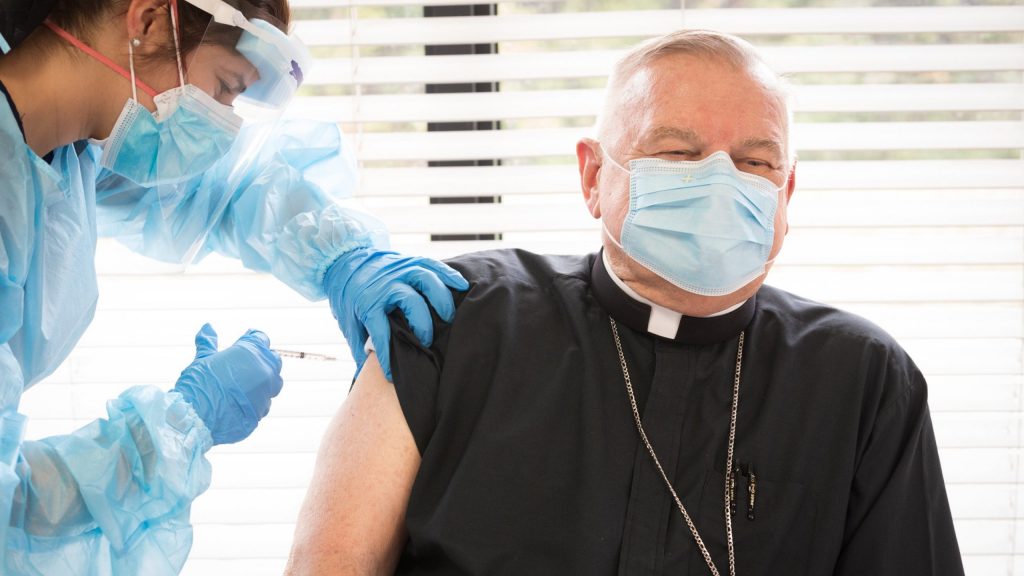
Admittedly not a big fan of needles and injections, Miami Archbishop Thomas G. Wenski winced as he became among the first South Floridians and possibly the first U.S. bishop to receive a COVID-19 vaccination.
Florida public health officials administered COVID-19 vaccinations Dec. 16 at St. John's Nursing Center, located near Fort Lauderdale on the north campus of Catholic Health Services of the Archdiocese of Miami.
It marked the start of a vaccination campaign that will initially extend to thousands of state health care workers, first responders and staff at nursing homes and other residential care facilities.
St. John's staff and nursing home residents were offered the Pfizer/BioNTech COVID-19 vaccine, which the U.S. Food and Drug Administration authorized for emergency use Dec. 11.
"I am always afraid of needles, and as a typical guy, I am not made for pain," Archbishop Wenski chuckled before being screened for and then injected with a dose of the vaccine in the lobby of St. John's Nursing Center.
The archbishop said he was there to set an example that the vaccination effort is a public good and a morally acceptable way for Catholics to help combat COVID-19.
"I wanted to show today, first of all, that we have confidence in the vaccine and that we don't have any ethical concerns about the vaccine," Archbishop Wenski told the Florida Catholic, Miami's archdiocesan newspaper. "Hopefully, my stepping up will encourage other people to get the vaccine as it becomes available to them."
"The bishops of the United States have issued a very detailed explanation of why we have no ethical concerns about these particular vaccines and we will encourage everyone to access them," he said.
"Even people who might not have much to fear from infection -- if they are not high risk and if they are healthy or if their demographic age is such that they would expect to recover easily -- the fact that they would get vaccinated is a benefit to others around them so that they might not be responsible for others catching the virus," the archbishop said.
Recently, the U.S. bishops addressed the moral concerns raised by the Pfizer and Moderna vaccines' connection to cell lines that originated with tissue taken from abortions in the 1970s.
However, this connection to morally compromised cell lines is remote, and the public health situation is too grave to reject the vaccines, said Bishop Kevin C. Rhoades of Fort Wayne-South Bend, Indiana, chairman of the U.S. Conference of Catholic Bishops' Committee on Doctrine, and Archbishop Joseph F. Naumann of Kansas City, Kansas, chairman of the USCCB's Committee on Pro-Life Activities.
Each state has a distribution plan for administering the vaccines. Moderna's vaccine was expected to receive FDA approval. National guidelines call for health care workers and those in nursing homes and long-term care facilities to be first in line to get immunized.
Nursing home and health care providers are in Florida's tier one group for the vaccination rollout.
"As we have learned before, other types of vaccines have done great things for protecting us over the years," Archbishop Wenski said.
Catholic Health Services, which operates 38 facilities in Broward and Miami-Dade counties, and other agencies of the Miami Archdiocese will encourage their staff and program residents to take a COVID-19 vaccine, he added.
The archdiocese has several thousand employees, residents and front line workers in archdiocesan nursing homes and rehabilitation hospitals, assisted living facilities, housing for the elderly, as well as Catholic schools staff and faculty who will soon have access to the vaccines, according to Archbishop Wenski.
"This is the first vaccination in the archdiocese that I am aware of," he added, noting that Jackson Memorial Hospital in Miami was host to another COVID-19 vaccination rollout this week.
Joseph Catania, president and chief executive officer of Catholic Health Services, or CHS, noted all residents and staff of the system's facilities will be among the first to receive the COVID-19 vaccine.
At St. John's Nursing Center, some 120 staff and 82 patients were being vaccinated Dec. 16.
"This is really a blessing, such a great Christmas present since CHS takes care of 5,000 elderly residents every day and their average age is 75 years old," Catania said. "And as everybody knows the mortality rate if someone in that age group catches COVID is 82%, so we are really so happy for Operation Warp Speed and the state of Florida in giving us this opportunity to get everyone vaccinated."
The Pfizer/BioNTech vaccine is two doses, with the injections separated by three weeks. The Florida Department of Health indicated the vaccine supply arrived Dec. 15, Catania noted, just a day before the health department and the Florida National Guard administered the vaccination at several stations at the CHS facility.
Catania said the agency has had a very strong infection control program that has allowed a safe means for family and loved ones to visit with relatives who reside at CHS elderly housing.
"Anyone who does contract COVID is separated from the general population and placed in an isolation area. We also have performed over 30,000 COVID tests over the last nine months for patients and staff," Catania said.
"Each of our staff have been tested 15 times over, and each of our residents have been tested 16 times during the last nine months, he said. "We have also screened 135,000 people coming into the facility during that time."
The government's Operation Warp Speed's goal is to produce and deliver 300 million doses of safe and effective vaccines with the initial doses available by January 2021, as part of a broader strategy to accelerate the development, manufacturing, and distribution of COVID-19 vaccines, therapeutics, and diagnostics.
Earlier this week, the vaccines moved from the Pfizer manufacturing facility to the UPS and FedEx hubs and then will go out to the 636 locations nationwide, according to U.S. Army General Gustave Perna, who is overseeing the government's vaccine program.
Distribution of the vaccine began Dec. 14, 24 hours after FDA approval of the Pfizer vaccine. Shipments of an FDA-authorized safe and effective COVID-19 vaccine are arriving at sites across America.