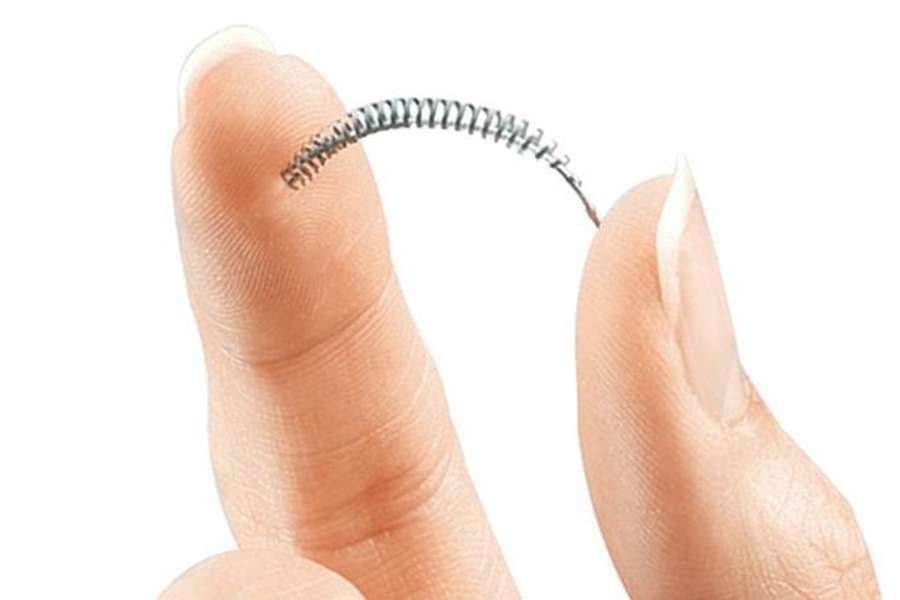
Washington D.C., Dec 1, 2016 / 03:02 am (CNA/EWTN News).- Angela Desa was 28 years-old when she got Essure, a form of permanent birth control made of flexible metal and polyester coils inserted into her fallopian tubes. The only form of permanent, non-surgical birth control, implanted Essure coils are supposed to stay in the fallopian tubes, where they create a chronic infection causing scar tissue to form, effectively closing the tubes and rendering the woman sterile.
At the time of Desa’s implants, the FDA warning on the device said that possible side effects could include “mild to moderate pain and/or cramping, vaginal bleeding, and pelvic or back discomfort for a few days.”
But the side effects Desa experienced were to the extreme. She felt like she had the flu constantly. The pain wasn’t mild or moderate, but debilitating and chronic. “They don’t tell you that it’s 'I can’t get out of bed and take care of my kids' kind of pain,” she told CNA in 2015.
Desa’s anger at her experience convicted her to join others in the fight against the device. Desa serves as one of several administrators for Essure Problems, a group of women who’ve had similar debilitating experiences with the medical device. They lobby against Essure in court, and provide support for women who have had issues with Essure, or who may be considering it.
Many of the women on the page have experienced even more serious side effects than Desa, including perforated organs due to coil migration, nickel allergies, fetal disfigurement due to nickel poisoning, chronic pain, exhaustion, increased risk of autoimmune disorders, and bouts of depression and suicidal thoughts.
Since CNA first reported on the group in February 2015, it’s membership has more than doubled - from 14,000 to more than 31,000. At the end of November, the group was able to celebrate a victory - the FDA officially placed its strictest warning, a so-called “black box” label, on the device. Black box warning labels are reserved for drugs or devices that are reasonably associated with very serious or even life-threatening health risks.
The decision to add the serious warning to Essure was announced in February. Since then, the FDA opened up a period for feedback during which they met with doctors and women who’ve had Essure implants to decide what should be included on the warning.
Holly Ennis is an attorney who works with the women from Essure problems. She met with the FDA and her clients several times to discuss concerns about what should be included on the warning before it was released.
One of her biggest concerns, she told CNA, was whether patients would actually see and know about the warning. Medical devices are typically shipped straight from their manufacturer to doctors or hospitals, leaving no way to ensure that patients would be made aware of the risks.
To that end, the FDA also asked Bayer to create a patient-doctor checklist, that lays out the risks associated with the device and has space for patients to sign as they go through the checklist. The checklist is not mandatory, however, and many doctors view it as burdensome or intrusive, according to The New York Times. Planned Parenthood told the Times that it plans on informing women of the risks associated with the device, but does not plan on having them sign the checklist.
“There’s no question there are complications, but there are risks and benefits to everything we do in medicine, and we don’t have good data to establish the magnitude of the problem,” Dr. Christopher M. Zahn, the vice president of practice for the American College of Obstetricians and Gynecologists, told the New York Times. “Decisions like these should be made based on data that’s appropriately vetted, not a series of anecdotal reports,” Dr. Zahn added.
But the women of the Essure Problems group would argue that they are the data - data that for a long time has been dismissed and ignored, Ennis said.
“We have 31,000 people on our page, probably 25,000 of them have had the device, have had this experience. Their frustration is no one is looking at their data. Instead the FDA is relying on a clinical trial that followed 200 people for two years for a device that is permanently implanted in the human body, as opposed to data from 25,000 women who are experiencing these symptoms every day,” Ennis said.
“Almost 7,000 women on the page have undergone the removal procedure (which almost always requires surgery, often a total hysterectomy). That is a lot of women. I don’t think people are voluntarily going in and getting hysterectomies if they’re not having a significant amount of discomfort,” she said.
The FDA has also ordered Bayer to conduct new clinical studies on Essure. The original clinical trials on the device were considered problematic by critics. No control group was used, and so some side effects experienced by the women in the clinical trials were dismissed as being caused by Essure.
Earlier this year, Bayer agreed to track 1,400 women with Essure over the next 5-6 years, though the study is already delayed. These women will be compared to 1,400 women who chose the sterilization method of laparoscopic surgery.
The Essure Problems women, however, believe that the device should be pulled from the market while clinical trials are still ongoing, Ennis said. “If you were a woman, would you sign up for this clinical trial?” Ennis said, given the debilitating side effects that thousands of women have experienced post-Essure. “They know that these women are suffering from these problems, and yet they’re going to continue to sell the device until Bayer proves it’s not safe,” she said.
The Essure Problems women were also concerned that the new FDA label still failed to mention some of the more common side effects experienced by women with Essure, including autoimmune disorders, brain fog, bloating, chronic inflammation, and foreign body reaction, Ennis added.
Nevertheless, the black box warning is an accomplishment for the grassroots organization of Essure Problems, Ennis said. And the women are not giving up. They will continue to fight, including lobbying Congress for laws that would get rid of pre-emption (which Essure received) and to improve medical device safety and medical device reporting from physicians.
“They’re not quitting, but they should be proud of what they’ve accomplished to date,” Ennis said. “And they won’t give up. I know these women, they’re very tenacious. They’re definitely not stopping.”